Peking University, May 18, 2020: A joint research team led by Sunney Xie, Director of Beijing Advanced Innovation Center for Genomics (ICG) at Peking University (PKU) has successfully identified multiple highly potent neutralizing antibodies against the novel coronavirus SARS-CoV-2, the causative virus of the respiratory disease COVID-19, from convalescent plasma by high-throughput single-cell sequencing. Generated by human immune system, neutralizing antibodies can effectively prevent viruses from infecting cells. New results from animal studies showed that their neutralizing antibody provides a potential cure for COVID-19 as well as means for short-term prevention. This marks a major milestone in the fight against the pandemic.
This study has been published online in Cell, titled "Potent neutralizing antibodies against SARS-CoV-2 identified by high-throughput single-cell sequencing of convalescent patients’ B cells ". This work was jointly conducted by Beijing Advanced Innovation Center for Genomics and Biomedical Pioneering Innovation Center of Peking University, Beijing YouAn Hospital of Capital Medical University, Institute of Laboratory Animal Science (ILAS) of Chinese Academy of Medical Sciences and Comparative Medicine Center, Peking Union Medical College, Beijing Institute of Microbiology and Epidemiology of Academy of Military Medical Sciences, Sino Biological, Inc., WuXi Biologics and Singlomics. The co-authors of this article are Yunlong Cao, Bin Su, Xianghua Guo, Wenjie Sun, Yongqiang Deng, Linlin Bao, Qinyu Zhu. The corresponding authors are Chuan Qin, Chengfeng Qin, Ronghua Jin, and Sunney Xie. The work which began on Jan. 27, 2020 was supported by The People’s Government of Beijing Municipality, the Ministry of Science and Technology and the Ministry of Education of the People’s Republic of China.


Sunney Xie (in the middle) and some members of his team
There has been an urgent need for highly effective drugs to cure COVID-19. Repurposed small-molecule drugs lack in specificity thus efficacy is compromised. Although plasma therapy has exhibited certain efficacy, it’s limited by convalescent plasma supply. The active component of plasma therapy is the target-specific neutralizing antibody. Antibody drugs as a kind of biologics have been successfully applied to treat viruses like AIDS, Ebola, and MERS. However, it is often time-consuming to develop neutralizing antibodies suitable for clinical use, taking months or even years.
By using their expertise in single-cell genomics, Sunney Xie’s team at ICG, PKU in collaboration with researchers of Beijing YouAn Hospital collected blood samples from over 60 convalescent patients, among which 14 highly potent neutralizing antibodies were selected from 8,558 antigen-binding IgG1+ clonotypes. Their most potent antibody, BD-368-2, exhibited an IC50 of 8pM and 100pM against pseudotyped and authentic SARS-CoV-2. Experiments on the authentic virus were completed in the P3 laboratory of the Academy of Military Medical Sciences.
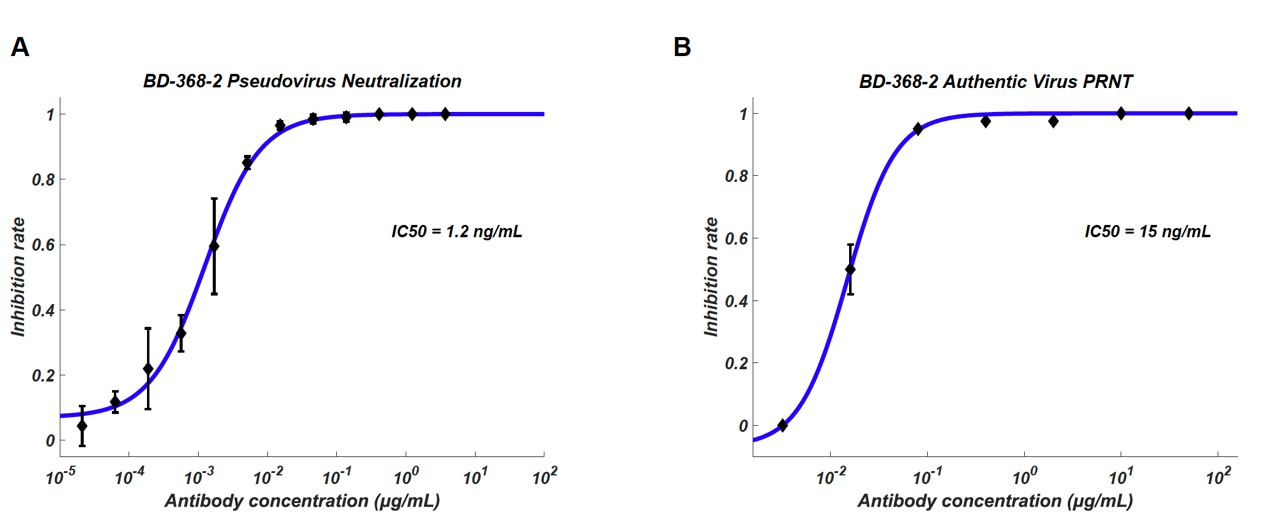
Neutralization potency of BD-368-2 antibody against pseudovirus and authentic virus, IC50 reached 8pM and 100pM respectively
The in vivo antiviral experiment of neutralizing antibodies has recently been completed, using hACE2 transgenic mice model developed by Dr. Chuan Qin’s lab at ILAS. The results showed that BD-368-2 antibody could provide strong therapeutic efficacy and prophylactic protection against SARS-CoV-2: When the BD-368-2 antibody was injected into infected mice, virus load was decreased by ~ 2400 times; when uninfected mice were injected with BD-368-2, they were protected from the virus infection.
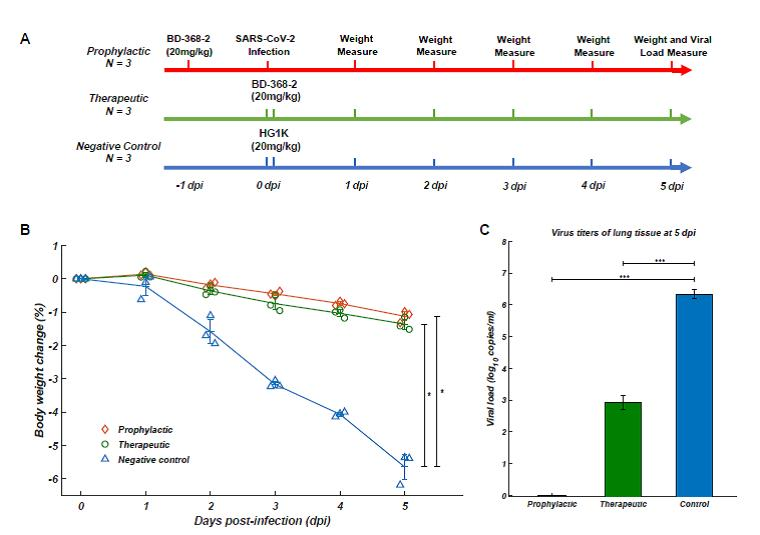
Testing on the therapeutic and prophylactic efficacy of neutralizing antibodies on mice models (A)Therapeutic group (green), injected with BD-368-2 two hours after infection (n=3); prophylactic group (red), injected with BD-368-2 one day before infection (n=3); control group (blue) injected with nonrelevant antibody two hours after infection.(B)The rate of weight loss of therapeutic and prophylactic groups was significantly lower than that of the control group.(C)After 5 days, the viral load of therapeutic group decreased by ~ 2400 times; no viral load was detected in the prophylactic group.
In addition, the structural biologists Xiaodong Su and Junyu Xiao and their group members in the PKU team also obtained the 3.8Å Cryo-EM structure of a neutralizing antibody in complex with the Spike-ectodomain trimer. It revealed the antibody’s epitope overlaps with the ACE2 binding-site, which provides the structural basis of neutralization. Moreover, they showed that SARS-CoV-2 neutralizing antibodies could be selected with high efficiency based on similarities of their predicted structures to those of SARS-CoV neutralizing antibodies, hence greatly expediting the screening process.
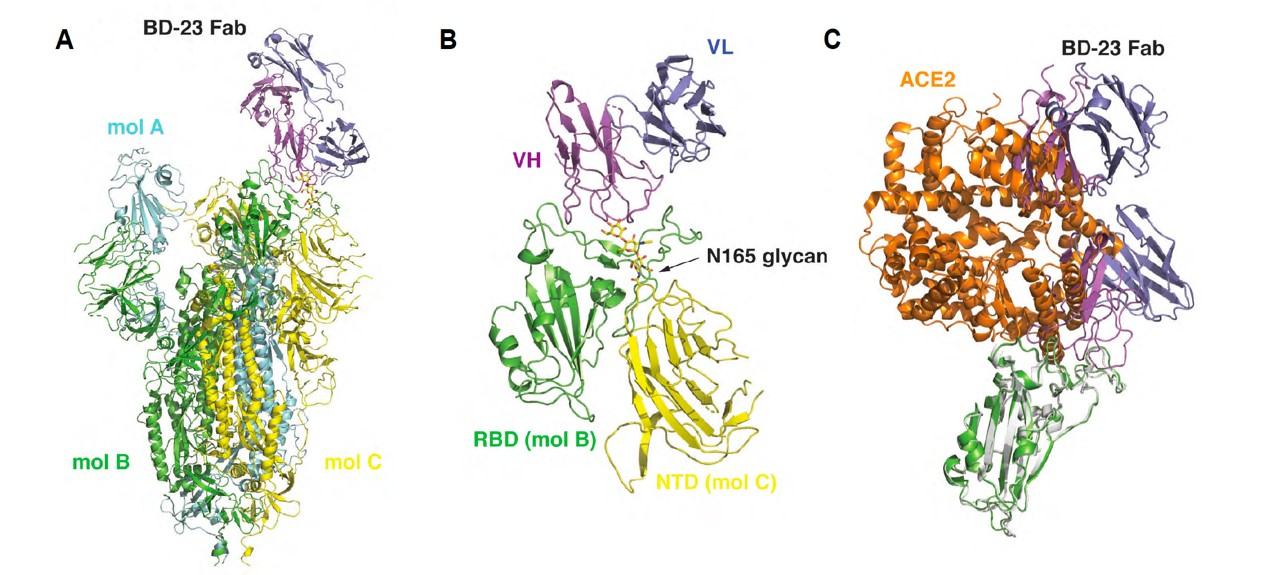
(A&B) Cryo-EM structure of BD-23 Fab in complex with the Spike-ectodomain trimer (C) RBD/ACE2 complex structure overlays with RBD/ BD-23 Fab structure, which demonstrates BD-23 Fab can block S protein binding with ACE2.
The potent neutralizing antibody could be used to develop drugs for both therapeutic intervention and prophylactic protection against SARS-CoV-2. Clinical trials are underway, and the research team have strong confidence in finding a cure. "If the COVID-19 epidemic reappears in the winter,” remarked Sunney Xie, “Our neutralizing antibody might be available by that time."
The paper can be accessed at https://www.cell.com/cell/fulltext/S0092-8674(20)30620-6