Peking University, Nov.19, 2012: Recently, Dr. Ying Jiang, from International Center for Quantum Materials of Peking University, and his co-workers open up the possibility of submolecular control of the bond-selective chemistry in a single functionalized molecule. This work is published in Nature Chemistry [Nature Chem. DOI: 10.1038/NCHEM.1488 (2012)], and the referee thinks highly of the work as an “experimental tour de force”.
Bond-selective chemistry, which involves selectively breaking and forming specific bonds, is one of the ultimate goals of chemistry. Achieving bond-selectivity in a complex functionalized molecule is particularly important for advancing the molecular nanoscience and technologies, such as molecular electronics, organic solar cells, and nanomachines. However, due to the rich internal degrees of freedom and the intramolecular energy redistribution in functionalized molecules, bond-selective control has so far been difficult to realize.
The team led by Dr. Ying Jiang, in collaboration with Dr. Qing Huan of Institute of Physics of CAS, Prof. Wilson Ho of UC Irvine, and Prof. Guillermo C. Bazan of UC Santa Barbara, succeed to induce a sequence of target-selective bond dissociation and formation steps in a single thiol-based π-conjugated molecule adsorbed on a NiAl(110) surface, using a scanning tunneling microscope (STM).
By locally injecting the energy-tunable tunneling electrons into the resonant states derived from the functional groups in the molecule, they are able to selectively abstract different functional groups from the molecule step by step and monitor the evolution of the molecular electronic structure both in energy and real space at each reaction step. Furthermore, the bond-selective dissociations allow them to activate the sulfur functional groups and form different types of Au-S bonds by manipulating and attaching a single gold atom to the sulfur atom each end of the molecule. The microscopic geometry of the Au-S bond at the single molecule level and its influence on the electronic structure of the molecule are determined by the STM, which may underlie the understanding of electron transport in the widely used thiol-based molecular junction.
This work also reveals the changes in the molecular electronic structure associated with bond dissociation and formation, which is crucial evidence for orbital hybridization in chemical transformation.
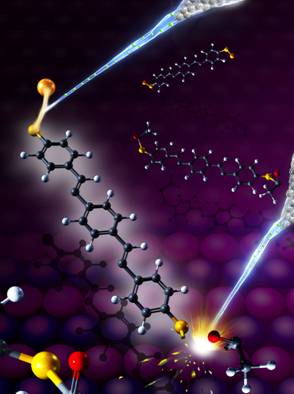
Figure: Selective bond dissociation and formation in a single functionalized molecule. The electron beams emitted from the tip of a scanning tunneling microscope (STM) selectively cleave the sulfur-acetyl bond (lower-right), and weld the sulfur-gold bond (lower-left) in a 1,4-bis[4’-(acetylthio)styryl]benzene molecule. The background is the NiAl(110) substrate, on which the molecule is adsorbed.
Edited by: Ji Fan, Zhang Jiang
Source: PKU News (Chinese)